zinc valence electrons|Zinc : Cebu To find the number of valence electrons for Zinc (Zn) we need to look at its electron configuration. This is necessary because Zn is a transition metal (d b. Home Pinay Sex Scandal Video Prinsesa sa ganda, . Prinsesa sa ganda, kinantot ng tambay. About. Date: December 6, 2023 Pinay Sex Scandal Video. Popular Videos More videos. Buti hindi nakakabulag ang pinutok sa kanyang mukha. Jackpot si Mark ang ganda ng natikmang dilag. Babe bilis baka madatnan tayo ni Tita.Looking for Intellicare accredited doctors in the Philippines? Book an appointment with a doctor that's accredited by your HMO provider here.
PH0 · Zinc Valence Electrons
PH1 · Zinc (Zn)
PH2 · Zinc
PH3 · Khan Academy
PH4 · How to Find the Valence Electrons for Zinc (Zn)
PH5 · How many valence electrons does zinc have?
PH6 · How Many Valence Electrons Does Zinc (Zn) Have?
PH7 · 3.1: Valence Electrons
PH8 · 3.10: Valence Electrons
PH9 · 10.6: Valence Electrons
Open Hours . Birdcage Bar. Monday to Friday from 5:00 pm until late Saturday & Sunday from 12:00 pm until late . LOCATION: Wrest Point Hotel & Casino – 410 Sandy Bay Rd, Hobart 7005 Tasmania, Australia. Call Us . Book Now . View All. Birdcage Bar . Fri, 06 Sep 2024. Velvet Divan. 7.30pm – 10.30pm. More .
zinc valence electrons*******To find the number of valence electrons for Zinc (Zn) we need to look at its electron configuration. This is necessary because Zn is a transition metal (d b. Readers can understand the valence electrons of zinc by referring to the dot diagram. The Lewis dot diagram explains and draws the numbers of valence electrons of Atom. The dot diagram shows the . The total number of electrons in the last shell and d-subshell after the electron configuration of zinc is called the valence electrons of zinc. The last shell of . Learn how valence electrons determine the reactivity and bonding of atoms. Find out the octet rule, the types of chemical bonds, and examples of valence electron .Learn how to identify valence electrons, the outer-shell electrons of an atom that determine its reactivity. See examples of valence electrons for representative and transition .zinc valence electrons Zinc Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The number of valence electrons .
How can you use the periodic table to find out how many valence electrons an element has? This article from Khan Academy explains the simple rules and patterns that can .zinc valence electronsZinc is a chemical element of the periodic table with chemical symbol Zn and atomic number 30 with an atomic weight of 65.382 u and is classed as transition metal and is . Zinc has only 2 valence electrons, both located in the 4s-orbital. This is based on its electron configuration and its ability to lose these electrons to form Zn2+ cation. See the detailed answer and .Glossary. Allotropes Some elements exist in several different structural forms, called allotropes. Each allotrope has different physical properties. For more information on the . Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) sublevel are called inner-shell electrons and are not involved directly in the element's reactivity, or in the formation of compounds. Lithium has a single electron in the second .Zinc is the 30th element in the periodic table and has a symbol of Zn and atomic number of 30. It has an atomic weight of 65.38 and a mass number of 64. Zinc has thirty protons and thirty-four neutrons in its nucleus, and thirty electrons in four shells. It is located in group twelve, period four and block d of the periodic table.
If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.The electrons that determine valence – how an atom reacts chemically – are those with the highest energy.. For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. [1] Thus, the number of valence electrons that it may have depends on the electron . Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd .
Introduction. T here are four principle orbitals (s, p, d, and f) which are filled according to the energy level and valence electrons of the element. All four orbitals can hold different number of electrons. The s-orbital can hold 2 electrons, and the other three orbitals can hold up to 6, 10, and 14 electrons, respectively.
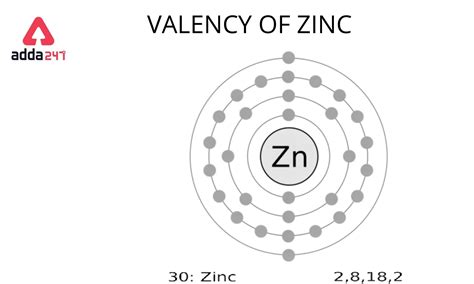
The valence electrons of Zinc are in the 4s orbital and there are two valence electrons. Since Zinc has two valence electrons, it can lose these two electrons to form a Zn 2+ cation, which has a stable configuration of 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰. Therefore, the valency of Zinc is +2. Orbital number of the subshell. The value of ‘l’ is from 0 to (n – 1). The sub-energy levels are known as s, p, d, and f. The orbital number of the s-subshell is one, three in the p-subshell, five in the d-subshell, and seven in the f-subshell. That is, there are thirty electrons in the atom of the zinc element. So, it is possible to determine the properties of zinc from the electron configuration. Now, the electron configuration of zinc shows that the last shell of zinc has two electrons. Therefore, the valence electrons of zinc are two. The last electron of zinc enters the d .
Valence electrons are the electrons located in the outermost energy level of an atom and are responsible for the element’s chemical reactivity. In the case of Zn, the valence electron configuration is [Ar] 3d 10 4s 2. This means that zinc has two valence electrons, occupying the 4s orbital.How can you use the periodic table to find out how many valence electrons an element has? This article from Khan Academy explains the simple rules and patterns that can help you determine the number of electrons in the outermost shell of any atom. You will also learn why valence electrons are important for chemical bonding and reactivity.Zinc Element Zinc (Zn), Group 12, Atomic Number 30, d-block, Mass 65.38. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element . Rôle biologique. Le zinc est vital pour tous les organismes vivants. Il forme le site actif de plus de 20 métalloenzymes. Le corps humain moyen contient 2,5 grammes de zinc et consomme environ 15 .

Group 12, by modern IUPAC numbering, [1] is a group of chemical elements in the periodic table.It includes zinc (Zn), cadmium (Cd), mercury (Hg), [2] [3] [4] and copernicium (Cn). [5] Formerly this group was named IIB (pronounced as "group two B", as the "II" is a Roman numeral) by CAS and old IUPAC system. [note 1]The three group 12 elements that occur .
The chlorine atom has the same electron configuration in the valence shell, but because the entering electron is going into the n = 3 shell, it occupies a considerably larger region of space and the electron–electron repulsions are reduced. The entering electron does not experience as much repulsion and the chlorine atom accepts an .Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .For the d-block and f-block elements, valency is determined not only on the basis of valence electrons but also on d and f orbital electrons. However, the general valencies of these d and f block elements are 2 and 3. . Valency of Zinc: 30: 2: Periodic Trends in the Oxidation States of Elements 1. Variation Of Oxidation State Along a Period.
June 15, 2009 – Charlotte, North Carolina (THREE 4 ALL) RAW Announcers: Michael Cole & Jerry “The King” Lawler ECW Announcers: Josh Mathews & Matt Striker SmackDown! Announcers: Jim Ross & Todd Grisham WWE INTERCONTINENTAL TITLE MATCH Chris Jericho defeated Rey Mysterio to retain the WWE Intercontinental title: Before the .
zinc valence electrons|Zinc